Enriched Oxygen Systems: Three Operation Best Practices

Three Operation Best Practices
Even though oxygen is abundant in our atmosphere (~21%), once we concentrate it above 23.5% it becomes a dangerous system media. Oxygen systems are dangerous not because oxygen itself is flammable, but rather because it increases the flammability of most materials around it. Things that we do not ordinarily think of as flammable, such as 316 stainless steel, become quite flammable in an oxygen-enriched environment.
Most of us know the three requirements for fire: oxygen (oxidizer), fuel and an ignition source. When working with Enriched Oxygen Systems, you will almost always have all three requirements present in your system. The only way to manage the risk of oxygen systems is to manage each of these three components by following design best practices. The most prominent and recognized standard on oxygen systems is CGA-4.4 Oxygen Pipeline and Piping Systems, which should be consulted for all matters related to oxygen best practices.

Without fuel, there can be no fire. Even materials you may consider to be nonflammable, are oftentimes flammable in elevated concentrations of oxygen. This is measured as the Oxygen Index, and is published in numerous online standards. For safe systems, designers aim to have a higher index value as this correlates to a higher concentration of oxygen required to continue burning the material at atmospheric pressure. For example, when considering material selection for seats & seals, PTFE is a safer bet than Buna-N or nitrile rubber because the PTFE has a much higher oxygen index and will require a much larger concentration to continue burning.
The third component of the fire triangle required to promote a burning is an ignition source and there are three major ignition mechanisms in oxygen systems:
Particle Impact
- Flammable particles: It is best to assume there is always going to be particles since it’s impossible to fully clean the system and prevent any particle ingress during fabrication, startup, or operation. To help mitigate this risk it is suggested to use burn resistant filters, and purge with an inert gas before startup.
- High Gas Velocity: commonly believed to be any velocity greater than 100 ft/s, where the kinetic energy of the particle is enough to ignite other materials.
- Severe Impact Angle: impacts of 45-90 degrees allow for the most efficient transfer of kinetic energy to heat so it is best to avoid geometry where this impact angle could be possible.
- Flammable Target: in any oxygen system, it is best to avoid materials that are flammable in concentrated oxygen and use materials such as brass or Monel which are less susceptible.
Adiabatic Compression
As gas is compressed, it produces heat. This can cause the temperature of surrounding materials to reach their auto ignition temperature resulting in a fire. To mitigate this risk, materials with higher auto ignition temperatures should be considered for any system where rapid pressurization is possible. Also to avoid using components that can cause rapid pressurization such as ball valves and plug valves.
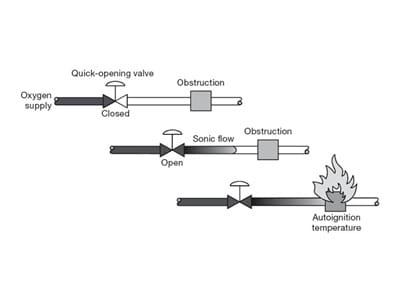
 Flow Friction
Flow Friction
Normally, only a risk in a high-pressure application, ignition is possible where a fast-moving stream flows past any non-metallic surface.
To reduce the risk of oxygen fires, focus on managing the three legs of the fire triangle: oxygen, fuel, heat. While we can’t remove the oxygen from the system, try to find ways to reduce the pressure of the system - a lower amount of oxygen molecules results in a lower risk to the system. To manage fuel, focus on compatible system materials that are burn-resistant such as brass or Monel and avoid metals such as stainless steel or aluminum. A good resource for information on compatible materials is NASA Technical Standard KTI-5210 or multiple online publications. Finally, focus on removing possible ignition sources or possible sources of heat such as adiabatic compression or particle impact as discussed above. This is best done by following industry best practices such as listed in (Safe Use of Oxygen and Oxygen Systems: Handbook for Design, Operation, and Maintenance): Second Ed.
